The Evolution of Tirzepatide’s Competitive Landscape
Recently, the Galien Foundation in the United States announced the winners of the Prix Galien USA Awards, often acknowledged as the “Nobel Prize of the pharmaceutical industry.” This prestigious award recognizes exceptional scientific innovations that significantly contribute to the improvement of human health and is considered one of the highest accolades in the pharmaceutical and biomedicine industries. Among this year’s winners, Ozempic from Novo Nordisk and Mounjaro from Eli Lily and Company both received this award for their breakthrough therapies!
Jump to…
An Overview View of Tirzepatide’s Top 10 Patent Assignees
Evolution of the Competitive Landscape
Access Comprehensive Data on Tirzepatide Sequence Patents for Free
What is Tirzepatide?
Mounjaro (tirzepatide) is a dual agonist for glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. It was approved by the US FDA in May 2022 for use in combination with diet and exercise to improve glycemic control in adults with type 2 diabetes. According to the press release of Eli Lily, Mounjaro represents the first new type of diabetes medication in nearly a decade.
A similar drug that’s recently gained popularity, Semaglutide (marketed as Ozempic) was approved by the US FDA in 2017 for the control of blood glucose in patients with type 2 diabetes. Semaglutide is a GLP-1 receptor agonist that stimulates the production of insulin and inhibits the secretion of glucagon, thereby reducing appetite and food intake. In June 2021, semaglutide injection (once weekly, 2.4 mg), marketed as Wegovy, was approved by the FDA for the first time for the treatment of obesity. This marked the first new weight loss medication approved by the FDA since 2014.
An Overview View of Tirzepatide’s Top 10 Patent Assignees
According to the Patsnap Bio Sequence Database, the top 10 applicants for the Tirzepatide patent are as follows:
- Eli Lilly with 101 applications
- Sun Pharmaceutical with 14 applications, and
- Applied Molecular Transport with 8 applications.
For more applicants, please refer to the image below.
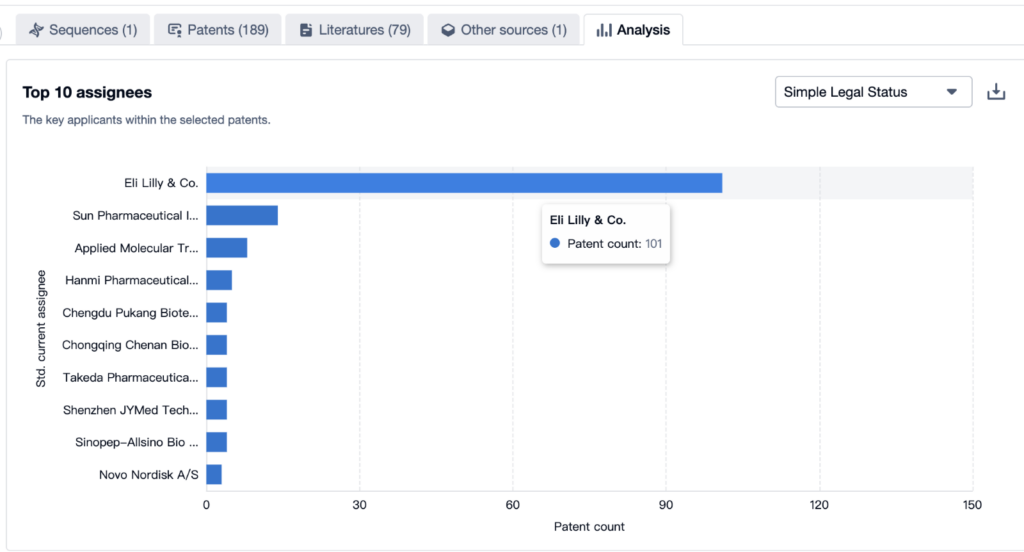
Source, Patsnap Bio
Evolution of the Competitive Landscape
Exploring the changes occurring within the application structure amongst tirzepatide’s top 10 patent assignees over time delivers insights into the competitive landscape’s evolution.
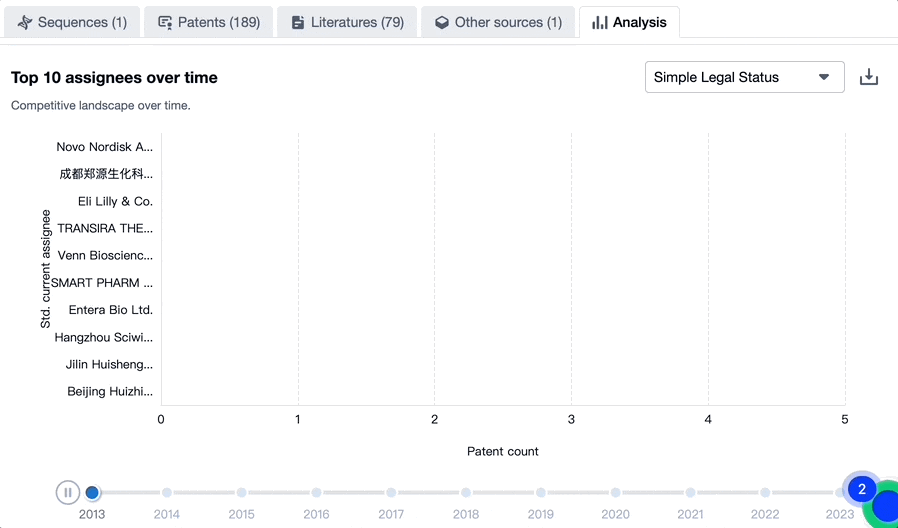
Source, Patsnap Bio
Access Comprehensive Data on Tirzepatide Sequence Patents for Free
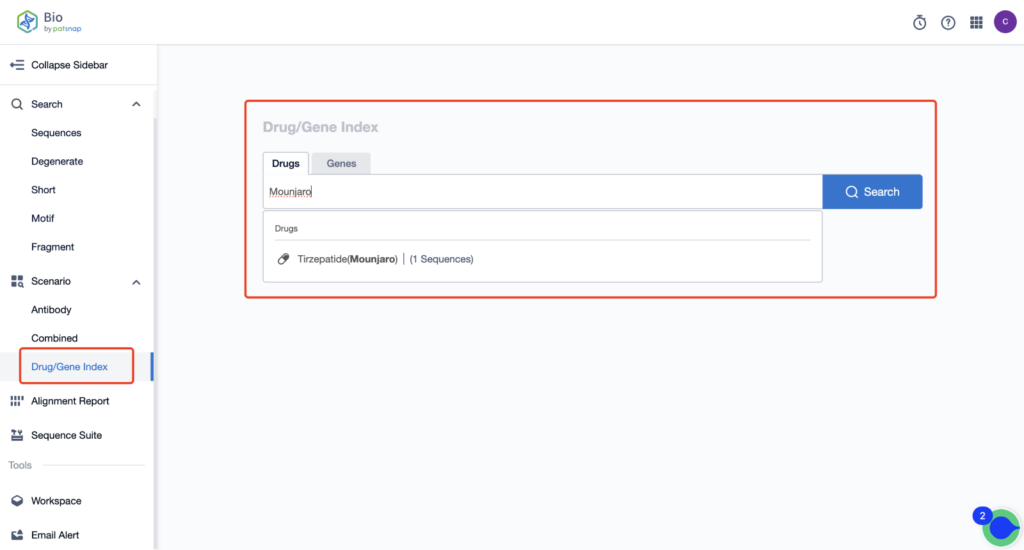
First, create a free account with Patsnap Bio Sequence Database. Then, navigate to the homepage’s “standard search” and enter the Tirzepatide sequence or simply input the drug name, Tirzepatide, in the “Drug/Gene index.” This step will uncover extensive information about Tirzepatide’s sequence, patent, literature, data from diversified sources, and visual representations of the competitive landscape of patents.
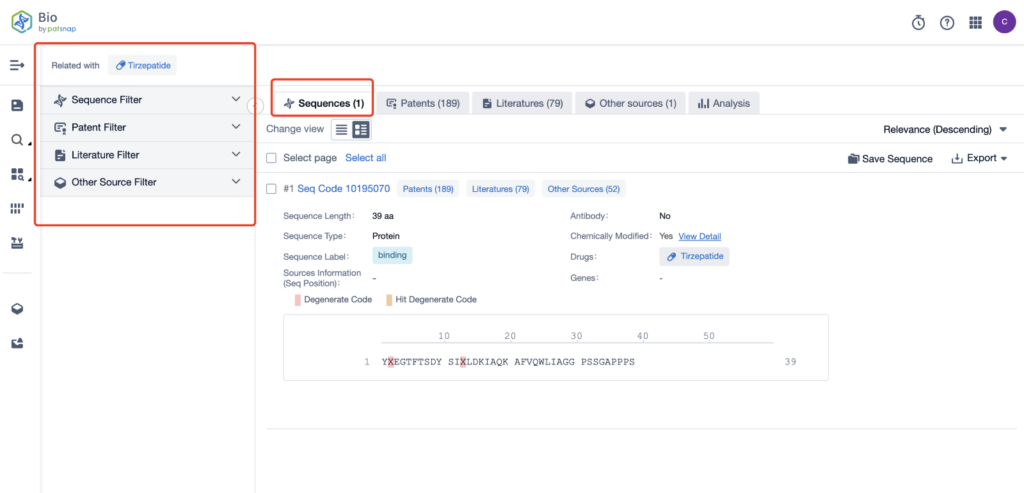
The left panel of the search results page is equipped with an array of filters that allow you to pinpoint specific data to enhance your search experience and overall efficiency. Clicking on each data point will reveal a comprehensive dataset and a host of practical tools to support your research.
Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated to highlight structural modifications to provide the most accurate sequence data and speed up the efficiency of sequence retrievals.
To get started, create an account today. Registration is free, so you have nothing to lose and so much insight to gain: https://bio.patsnap.com.