Life Science: Sickle Cell Disease News & Investments
Sickle Cell Disease (SCD) is a group of inherited red blood cell disorders. It’s caused by a genetic defect and occurs when both the mother and father pass down a defective gene.
We curated this article using insights from our Synapse platform. Keep track of advancements in drug developments and clinical trials when you register for Synapse.
Our red blood cells contain a protein called hemoglobin, which is responsible for carrying oxygen throughout our bodies via the cardiovascular system. Normally, red blood cells have a smooth and disk-shaped appearance; with SCD, the red blood cells are stiff and sickle-shaped, limiting the amount of oxygen they’re able to hold and transport and making movement through blood vessels difficult. The sickle shape of the red blood cells also decreases their lifespan: instead of the typical up to 120 days with normal cells, sickle cells die almost 10 times faster, living for just 10 to 20 days. There are many prominent symptoms of SCD, with a few on the list including:
- Anemia, which is lower-than-normal iron levels in the blood.
- Sickle crisis, meaning that the sickle-shaped cells clump together and cause blood vessel blockages that preclude blood from reaching muscles and organs.
- Stroke, with the ischemic type (blockage in blood flow) far more common in SCD than hemorrhagic (uncontrolled bleeding) strokes.
- Jaundice, where the sickle cells die faster than the liver can filter them out, allowing toxins to build up in the body. This causes the skin and whites of the eyes to become tinged yellow from the broken-down bilirubin that builds up from lack of clearance.
Currently, there is no cure for sickle cell, but research and development (R&D) advancements are underway. In this article, we explore news and investments being made in this space.
Sickle Cell News and Investments
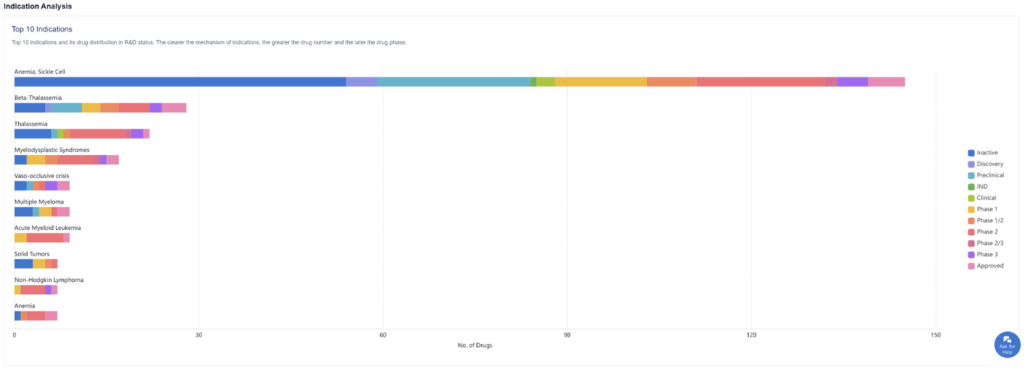
Many indications of sickle cell disease can warrant various drugs and treatments, as we can see in the graph above. Sickle cell anemia currently has the most activity in terms of approved drugs compared to their competitors, as well as drugs in later drug phases.
Treatments are advancing and some big players in the industry are feeling the pressure to get into the indication space through acquisitions. One notable acquisition recently is Pfizer acquiring Global Blood Therapeutics for $5.4 billion USD to obtain their pipeline on exciting and promising SCD treatments, some of which include a very key asset drug, GBT601 (a sickle hemoglobin polymerization inhibitor), which is potentially a “functional cure” for SCD. There is much hope and promise surrounding GBT601, as it is currently in the middle stage of a phase II/III trial for SCD. It is a once-daily therapy that can reduce the destruction of red blood cells as well as reduce the occurrence of a vaso-occlusive crisis, which is when red blood cells deprive tissues of oxygen by blocking blood flow. This acquisition adds an extensive pipeline of SCD treatment to Pfizer.
Pfizer is not the only large pharmaceutical giant to be making investments in its pipeline for SCD. In recent news, Novo Nordisk announced it will buy Forma Therapeutics in a deal valued at $1.1 billion USD. This deal marks two major acquisitions in the sickle cell disease space in just 30 days. The second acquisition leads to Novo Nordisk acquiring Forma’s lead drug candidate in the SCD space, Etavopivat. Etavopivat is a selective pyruvate kinase-R activator made to improve anemia and is taken once daily, just like GBT601. As mentioned by Novo Vice President Ludovic Helfgott, this acquisition is not “out of the blue”, as the company is making a large commitment to creating treatments for hematological disorders. The attraction to acquiring Etavopivat is due to its great safety profile and efficacy, its once-a-day usage, and the capability to partner this drug with other treatments.
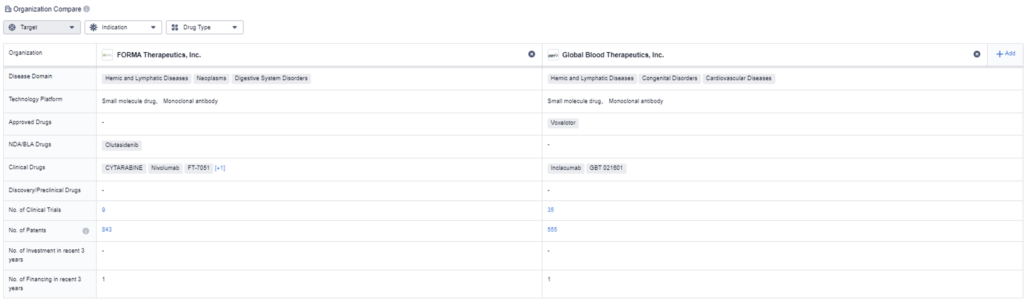
How do these two acquired companies stack up against one another? If we look at approved clinical drugs, Forma currently has four and Global Blood Therapeutics has 1. However, Global Blood Therapeutics does have significantly more clinical trials; currently, they have 35 ongoing trials, compared to Forma, which only has nine. We can conclude that both acquisitions look favorable for both Pfizer and Novo Nordisk as they are inheriting strong pipelines.
Conclusion
We can expect more investment and advancement in sickle cell disease with both acquisitions mentioned above. These investments and drugs provide hope to individuals looking for treatment for SCD. If you are interested in learning more about this space or keeping track of drug development and clinical trials, try Synapse for free.
Your recommended content
-

Patsnap Surpasses US$100 Million in Annual Recurring Revenue
Category: Article | Category: News/PR
Wednesday, June 12, 2024
Patsnap has reached a significant milestone of achieving $100M in Annual Recurring Revenue (ARR), marking an impressive 20% year-over-year growth in 2023. This milestone highlights the massive and meaningful value our platform brings to over 12,000 IP and R&D teams across 50 countries, driving efficiency, productivity, and collaboration.
-

Introducing Hiro, an AI assistant built for IP and R&D workflows
Category: AI advancements | Category: AI development | Category: AI-tools | Category: Article | Category: artificial intelligence
Tuesday, May 14, 2024
Powered by Patsnap’s industry-specific LLM, Hiro is designed to streamline IP and R&D workflows from ideation to product launch. With its robust AI capabilities, Hiro brings a new level of efficiency, precision, and security to tasks that were once time-consuming and labor-intensive.What sets Hiro apart is that it draws from our large language model that’s been trained on market-leading patent records, academic papers, and proprietary innovation data. This ensures we deliver more accurate and reliable results for every prompt.
-
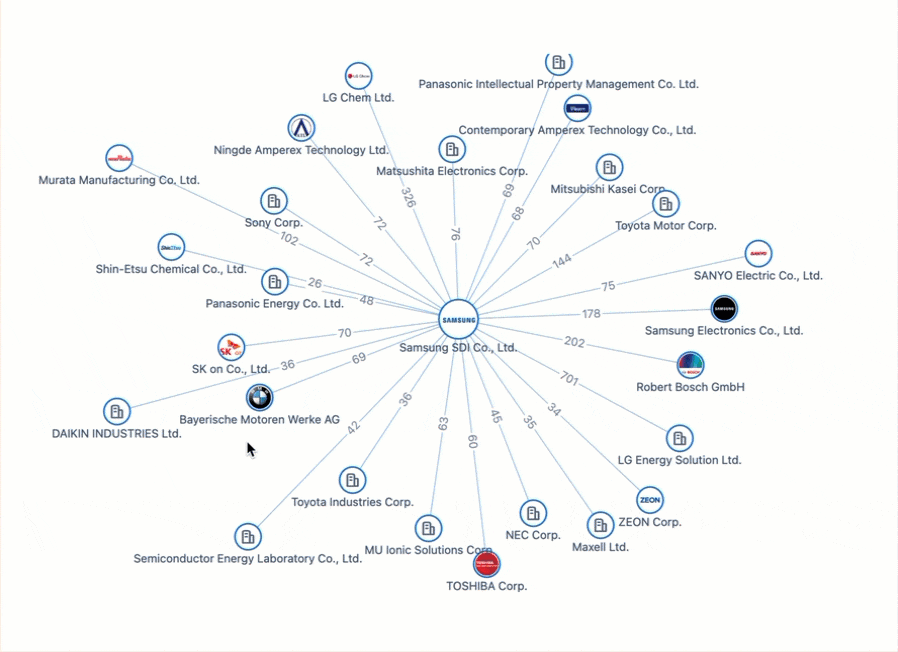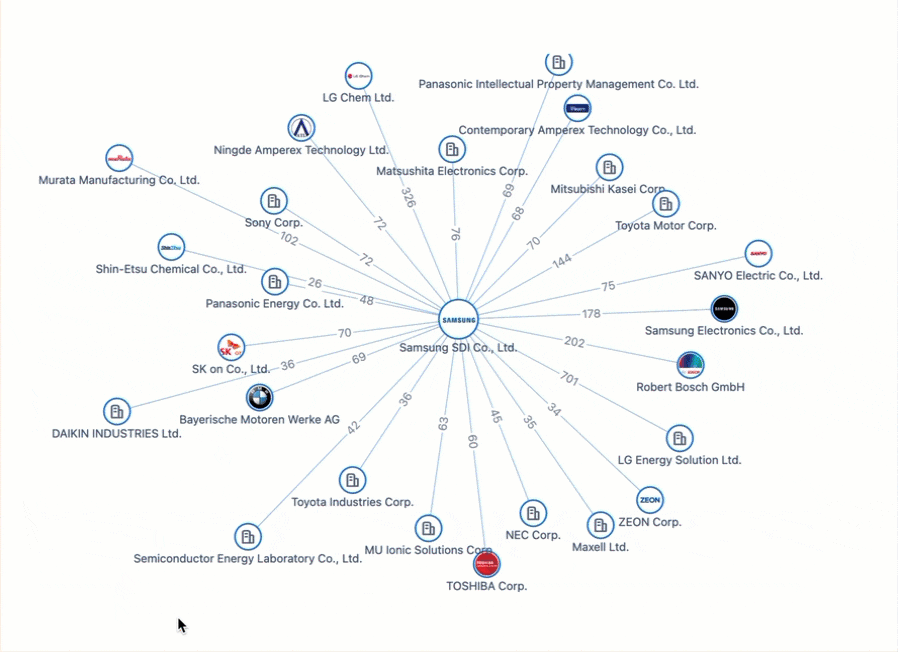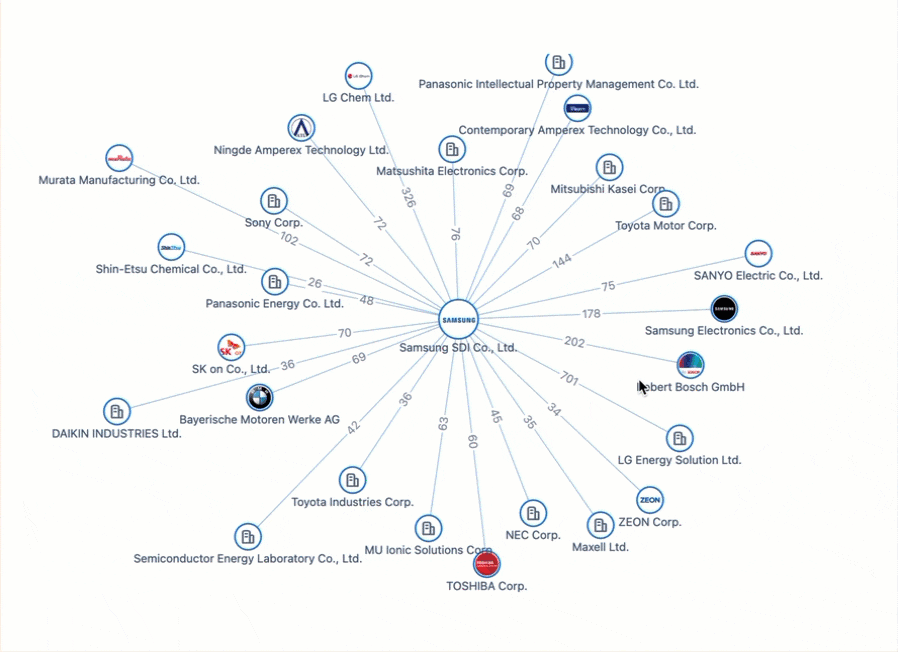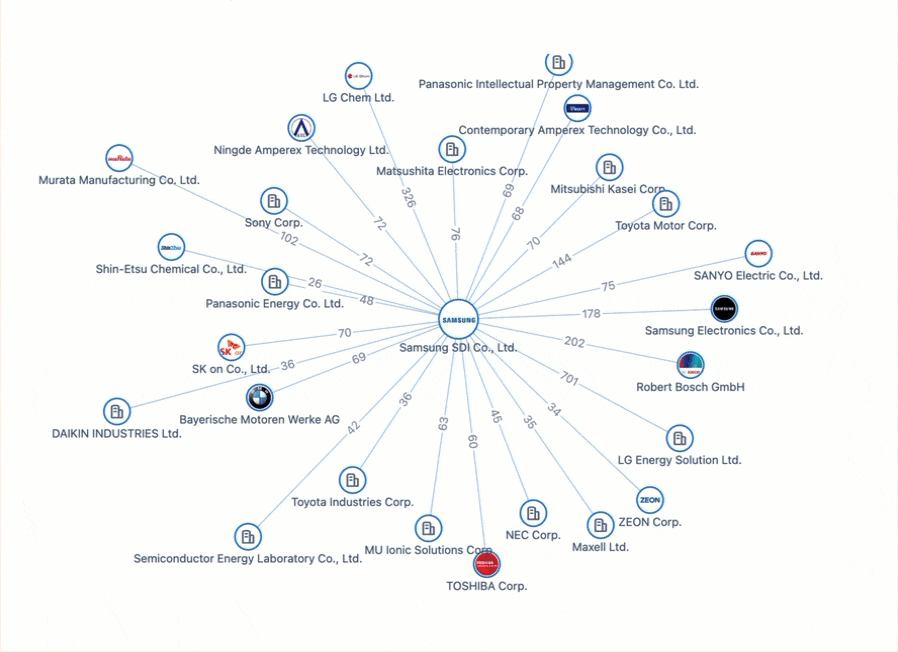
Powering the Future of Electric Vehicles: The Battle for Battery Innovation and Patents
Category: Article | Category: battery technology | Category: electric vehicle | Category: EV | Category: lithium ion | Category: lithium ion battery | Category: NEV | Category: new energy vehicles
Monday, April 22, 2024
In the ever-evolving landscape of innovation, the electric vehicle (EV) industry stands as a beacon of technological transformation. As we explore the patents propelling the EV revolution, Apple's venture serves as a poignant example of the challenges even industry giants face in this competitive arena. Join us on a journey through the global patent landscape, where the quest for superior power solutions unfolds, and where the true pioneers of the EV revolution are making their mark.